The European Union's medicines regulator has recommended the Pfizer-BioNTech coronavirus vaccine for use in the bloc's 27 states.
The European Medicines Agency (EMA) authorised the drug for the EU's nearly 448 million inhabitants after it went into circulation in the UK and the US.
Hours after the EMA's decision, the European Commission gave its own formal approval for the use of the jab.
Distribution could begin in some EU states as early as Sunday.
More than 311,000 lives have been lost to the pandemic across the bloc.
Fears of a new surge in cases among people gathering for the Christmas and New Year holidays have prompted partial lockdowns in countries like Germany and the Netherlands.
The threat of a new, more infectious Covid-19 variant spreading from the UK has also seen several EU states suspend travel from Britain.
How did the EMA rate the vaccine?
The agency said the drug had demonstrated an efficacy of 95% and could be used in people aged 16 and over.
"Today's positive news is an important step forward in our fight against this pandemic, which has caused suffering and hardship for so many," said the EMA's executive director, Emer Cooke.
Ms Cooke added: "Our thorough evaluation means that we can confidently assure EU citizens of the safety and efficacy of this vaccine and that it meets necessary quality standards.
"However, our work does not stop here. We will continue to collect and analyse data on the safety and effectiveness of this vaccine to protect people taking the vaccine in the EU."
Germany is setting up several hundred immunisation centres, and hopes to administer the first doses to its most vulnerable citizens on Sunday 27 December.
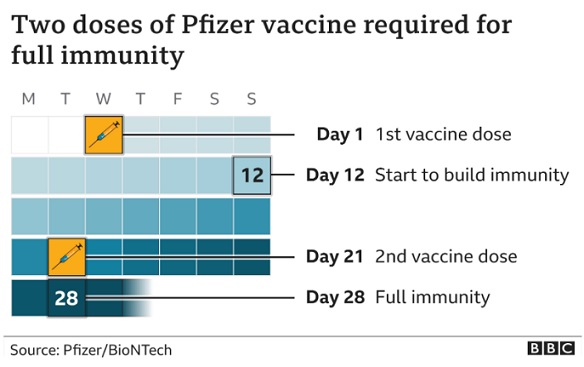
The vaccine is given as two injections, 21 days apart, with the second dose being a booster. Immunity begins to kick in after the first dose but reaches its full effect seven days after the second dose.
Most of the side effects are very mild, similar to those after any other vaccine and usually last for a day or so, said Prof Sir Munir Pirmohamed, chairman of the UK's Commission on Human Medicine expert working group, in an interview earlier this month.

